Analysis of B and T cell immune repertoire in chronic inflammatory diseases
T cell immunity is mediated by membrane bound T cell receptors (TCR) that interact with peptide antigens presented by MHC molecules. During T cell activation only a few of the million CD4 T cells express by chance a TCR with specificity for the presented peptide-antigen:MHCII complexes. After binding of the TCR to the peptide-antigen:MHCII complex, T cells clonally expand and differentiate into effector T cells. Since these T cell subsets determine the outcome of diseases it is of great interest to find out how pathogenic T cells are formed. One possibility to follow the formation of pathogenic T cell clones is to clonally track changes in the TCR repertoire during the course of diseases. This can be achieved by identifying the sequences of the hypervariable CDR3 regions of all TCR genes in one individual by Next Generation Sequencing (NGS). To establish the method and to test the sensitivity of this approach, T cells of wild type mice (Fig. 1A) and transgenic OTII mice (Fig. 1B), which hold only T cells specific for ovalbumin peptide, were mixed in the proportions of 1: 0.1 (Fig. 1C) and 1: 0.001 (Fig. 1D). As shown in Fig. 1 OTII specific T cells can be identified at a frequency as low as 0.01 %.

Figure 1. Tree Map of sequenced CDR3 regions of T cells from wild type mouse (A), OTII mouse (B), wild type with 10% OTII mouse (C) and wild type with 0.01% OTII (D). Each spot represents a unique entry.
All samples were prepared and analyzed according to the manufactures protocol of iRepertoire™ Inc, Huntsville, USA.
In autoimmune diseases, which are driven by pathogenic autoantibodies, the analysis of hypervariable B cell receptor sequences with NGS allows identification of autoreactive B cell clones. Before sequencing the variable gene repertoire, autoreactive B cells are identified by flow cytometry and sorted by binding to their autoantigen (Figure 2).
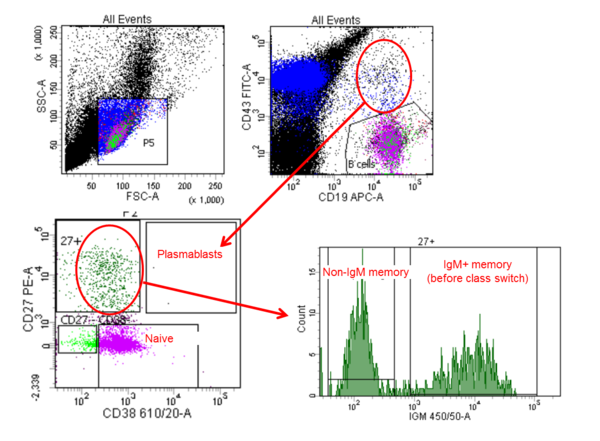
Figure 2.
The assessment of the B and T cell receptor repertoire opens a new possibility to identify pathogenic and autoreactive B and T cell clones in patients with high sensitivity and high throughput.
Principle Investigator
Dr. Kathrin Kalies
(Institute of Anatomy)
Dr. Andreas Recke
(Department of Dermatology)


